The over-arching goal of the lab is to understand how tissues and organs are sculpted in the developing embryo through an integration of genetic, molecular, and biophysical cues. Using live in vivo imaging, gene misexpression, and a combination of experimental and computational biomechanics in a classical model of embryology - the chick embryo - my lab aims to understand how forces that shape the embryo are specified by developmental signals, and how these forces in turn feedback on cell behaviors underlying growth and morphogenesis.

Biomechanics of intestinal looping
Buckling due to differential growth represents one of the core physical mechanisms driving morphogenesis throughout the vertebrate embryo. One example of this is looping of the small intestine. Looping permits proper placement of the lengthy small intestine within the confines of the body cavity, and results from buckling of the gut tube as it elongates against the constraint of a thin, elastic membranous tissue, the dorsal mesentery. Recently, we have identified BMP signaling as a crucial mediator of differential growth in the intestine, and demonstrated that tuning BMP activity in the intestine can coordinately increase or decrease buckling. BMP-mediated changes in the number and curvature of loops can be entirely predicted from experimentally measured growth rates, geometry, and mechanical properties. This project uses looping as a model to study the feedback between molecular cues and mechanical forces in driving buckling morphogenesis.
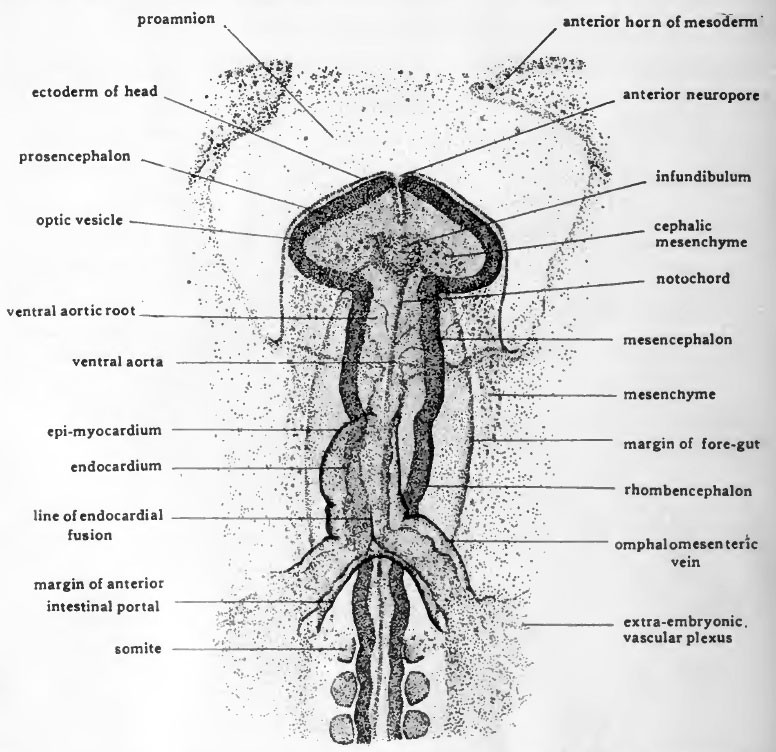
Mechanobiology of early brain development
Despite the profound complexity of the central nervous system, its embryological origin is seemingly simple: a cylindrical tube inflated internally by cerebrospinal fluid (a "thick-walled pressure vessel" in engineering terms). This project aims to investigate how neuroepithelial progenitors in the brain tube integrate mechanical stimuli with developmental signals to control growth, morphogenesis, and differentiation.

Molecular control of forces driving endodermal morphogenesis
The endoderm begins as an epithelial sheet on the surface of the embryo that becomes internalized to form the gut tube. The gut tube goes on to form the lining of the entire respiratory and gastrointestinal tracts. Although it is a fundamental aspect of establishing the adult body plan, how the endoderm is internalized and transformed from a sheet into a tube has been severely understudied. This project employs live in vivoimaging, strain and force measurements, and gene misexpression to understand how genetic and molecular cues coordinate morphogenetic forces to form the vertebrate foregut, midgut, and hindgut.